Extreme Ice Cream

01
Introduction
Molly:
I have been a reporter and editor for years and have done my fair share of in-depth research into niche subjects, especially at academic conferences. So when Dan and I snagged two spots at the world’s most prestigious ice cream science course, I was psyched. A week devoted to all things ice cream—what could be better?
While the short course was not the ice cream–eating binge I expected, it provided a deep dive into the science of commercial ice cream production. It also gave us an inside look at the people who make ice cream on an industrial scale and the food industry that defines the typical American summer.
Dan:
I’m the ice cream guy in our test kitchen. I developed my first recipe for a classic custard vanilla flavor five years ago, and I’ve been playing with my own recipes ever since. I’ve dreamed of attending the Penn State Ice Cream Short Course ever since I started scratching the surface of ice cream science. When Molly and I landed seats at the course, I hoped that I’d finally unlock the secret to making perfect ice cream at home.
The course didn’t disappoint. But it did disprove much of what I thought was true about ice cream. As it turns out, ice cream as a commercial commodity is the perfect intersection of science, marketing, and old-fashioned American ingenuity.
02

The Beginning
On a bitterly cold Sunday in January of this year, we joined 133 ice cream professionals as they filtered into a conference room on the Penn State campus, in State College, Pennsylvania.
We arrived right at noon, in a rental SUV that we had driven from the Philadelphia airport. At reception, we received a tote bag with a massive binder of lecture notes, a 500-page course manual, and badges bearing our names. We took our seats in the large lecture hall that would be our home for the next week and listened as each attendee introduced him or herself, one by one, on a microphone passed around the room. There were flavorists, plant managers, scoop shop owners, entrepreneurs, and chefs. There were R and D experts, PhD candidates, production managers, and us.
A palpable energy filled that florescent-lit conference room. After all, this was the Penn State Ice Cream Short Course.
In its 124th year, the Penn State Ice Cream Short Course is legendary. It’s attracted thousands of great ice cream makers over the years. And it continues to attract more hopefuls. Even though this year’s course clocked in at $2,160 per head (not including lodging), it filled up six months in advance. By January, the waiting list had 200 names.
Why would someone pay more than two grand to study ice cream for a week in the middle of January? For us, the answer was simple: to finally understand one of the world’s most complicated foods through the lens of science. Having spent years developing recipes and striving to understand how food works in the kitchen, creating professional-quality ice cream at home has remained the holy grail. Could we infiltrate the world of commercial ice cream, learn its secrets, and bring that knowledge back to the test kitchen to make better recipes?
There were surprises during the week we spent in State College. Some small, some large. The first came when we voted on our class name and “The Whip ’N Whey Naes” won. Second was the simple fact that industrial ice cream is really different than homemade ice cream. Of course we knew that. But we hadn’t realized just how different it actually is.
03

What is Ice Cream?
Ice cream is complicated. And not just for home cooks who try to make a batch of creamy vanilla, only to end up with a hard icy quart that pales in comparison to the stuff at the local scoop shop. It’s also complicated for food scientists. When home cooks think about making ice cream, their shopping list looks something like this: milk, cream, sugar, and eggs. The commercial producer, on the other hand, calls upon dozens of ingredients that offer complete customization.
And big producers don’t just rely on a trusty recipe, they use technology to perfectly balance ratios of fat, protein, sugars, emulsifiers, and stabilizers. Their goals? To produce a consistent product that’s still smooth and creamy when you pull it out of your home freezer, to create appealing flavors, to maintain a price point, and to keep from killing anyone with Listeria while they’re at it. Beyond nailing the mix ratio, they need to perfect the processes of homogenization and pasteurization, freezing and hardening, and packaging and storing. To make great supermarket ice cream, one must contend with microbiology, chemistry, physics, and law.
But before they took us too deep, the course instructors started at the beginning.
What, exactly, is ice cream?
Ice cream is an emulsion, or a combination of two liquids that do not want to mix. In this case, fat and water. Ice cream is also a foam; it contains a mass of tiny air bubbles within it, incorporated though agitation. And ice cream is a multiphase system, meaning it contains many different phases at once: in this case, solid, liquid, and gas. Legally, to be called ice cream in the United States, it must be frozen while stirring. It must not contain less than 1.6 pounds of total solids per gallon. It must not weigh less than 4.5 pounds per gallon, or contain less than 10 percent milk fat, and 20 percent “milk solids not fat” (more on this oddly named ingredient follows).
In other words: We had a lot to learn.

04

Ice Cream History
Ice cream, despite its fleeting nature and need for freezing temperatures, has been around for a long time. Various myths exist about its origin, including that it was brought ashore by Marco Polo or invented by Catherine de’ Medici. As it often is, the truth is far more nebulous.
We do know that ice cream began appearing regularly in America in the 18th century, an elite treat whipped up for special occasions for the likes of George Washington, Thomas Jefferson, and Governor William Bladen of Maryland. It was in the early 1800s that things changed, due to the invention of insulated icehouses. Suddenly ice cream needn’t be such a luxury. In 1851, Jacob Fussell, a milk dealer in Baltimore, began manufacturing ice cream commercially.
The Penn State Ice Cream Short Course debuted in 1892, when the college offered a free dairy manufacturing class during the winter. The ice cream course became its own, separate offering in 1925, when the culture of ice cream making was in the midst of change, due to the use of dry ice for ice cream transportation and the invention of the home freezer. (In 1920, ice cream was “generally recognized as a protected and essential food,” as W. S. Arbuckle wrote in his seminal book called, not surprisingly, Ice Cream.) Today, the course is sponsored by the Department of Food Science at Penn State and is currently led by Dr. Robert Roberts, a genial, generous professor with short brown hair and wire-rimmed glasses.
Roberts has a background in dairy chemistry, though ice cream was not a huge part of his career until 1999, when Penn State asked him to take over leadership of the short course. “Ice cream became an opportunity,” he told us, while sitting in an empty hotel room during a break in the course. “Within two days of taking over the ice cream course, I was getting calls. ‘Can you make this?’ ‘Can you help us with that?’” Compared with the slower pace and struggles of the yogurt-culture community, where he had been spending most of his time before steering the short course, this was invigorating. “You begin to realize people are hungry for the energy here,” he said.
We wondered what made this niche short course, of all the niche food courses out there, so popular. Why ice cream?
Roberts put it simply: “It has a lot of positive memories associated with it.” In other words, ice cream is fun.
This positive association is one of the more enduring qualities of ice cream, he added, especially when it comes to sensory evaluation. “Even crappy ice cream is great.”
The goals of the Penn State Short Course have changed over the 17 years Roberts has led the course. This is due, in part, to the fact that the ice cream industry has changed. In 1970, there were 3,749 dairy plants manufacturing one or more dairy products, while in 2015, there were 1,267, according to the USDA Dairy Products report. This consolidation has in turn changed the makeup of the ice cream course population. There just aren’t as many manufacturers. “So we get manufacturers, and we also get entrepreneurs,” said Roberts. “I think we meet the needs of both.”
Jeni Britton Bauer, founder and owner of Jeni’s Splendid Ice Creams, a successful artisanal ice cream shop from Ohio that now has national distribution, attended the short course in 2000. When she was there, she said, it was mostly men, all of whom worked in ice cream plants. Every year, a class photo is taken of the participants in the short course, a tradition that harks back to the early 1900s. She described the class picture taken her year with a laugh: “Little me, one other woman, and a sea of men with mustaches.”
In the years after she took the course, Bauer went on to scale her business for national distribution, while continuing to make all her ice creams completely from scratch, using dairy from grass-fed cows and making innovative flavors. “Entirely due to our success in the ice cream business,” she explained, “we’ve revved up and revived the interest in [course] attendance from smaller ice cream companies.”
But, she added, “it’s really not the place to learn how to make ice cream; it’s where you learn the science of ice cream.” As a scoop shop owner, “you don’t really need that—you start with a base and then you flavor it.”
And that, right there, was one of the biggest surprises at the short course: the simple fact that most ice cream makers start with a base that they buy from a larger company.
“Everyone does it, even the artisanal ice cream makers,” said Bauer. “Everyone in San Francisco is using Straus Family Creamery mix. I’ve never met anyone who is not.”
05
What’s in the Mix?
There are many, very good reasons why most ice cream shops buy ice cream base from companies like Hood, Nestlé, or Straus Family Creamery. Base mixes are a blank slate that can be easily flavored and customized, they come in a range of fat contents, and they’re convenient. We learned that the same commercial base made with 14 percent fat can make an ice cream that is dense and rich—or light and airy—depending entirely on how the base is frozen.
But the biggest reason scoop shops buy commercial base? They’re homogenized and pasteurized. That might not sound like a big deal, but both techniques are critical for the success of a commercial ice cream. Homogenization dramatically reduces the size of the fat droplets in the mix, allowing them to freeze into a fine network that traps the air incorporated during churning, creating a smooth, creamy ice cream. Pasteurization denatures the whey proteins found in milk, allowing them to trap more water and reduce iciness. But more important, pasteurization kills bacteria and is a requirement for the legal sale of ice cream in the United States. You can’t homogenize without an expensive homogenizer, and while there are other ways to heat your base to temperatures high enough to kill harmful microorganisms, the law doesn’t consider it pasteurization if you don’t have the required equipment. For entrepreneurs and small business owners, the costs to bring these processes in-house are prohibitive. The manufacture and distribution of commercial base is, in many ways, the very reason why artisanal ice cream shops have been able to proliferate and grow.
So what goes into a commercial ice cream base? While the inputs can vary based on which products are available and at what cost, the basic components of the mix are always the same. Every ice cream base is a combination of water, fat, sugar, milk solids not fat (more on this oddly named ingredient follows), and often stabilizers and emulsifiers. Custard bases contain egg yolks, too, which contribute water, protein, fat, and the emulsifier lecithin.
WATER
Because the majority of milk is water, all ice cream bases are also mostly made from water—and that water is incredibly important. In ice cream, water acts as a solvent and a dispersant. And, of course, as it freezes, liquid water turns into ice and the size of the ice crystals determines whether or not your finished ice cream feels icy or not. The amount of ice formed determines not only how hard the ice cream is, but also how cold it feels in your mouth.
FAT
Chemically, milk fat is a mixture of different triglycerides, and it exists as tiny, tiny droplets called globules. A single drop of milk contains 100 million globules. Through homogenization, these fat globules are broken down into even tinier particles and surrounded by casein (one of the proteins found in milk), which help keep them separated. During freezing, these fat globules harden, stick together, and form a network that traps air.
SUGAR
Sure, sugar (sucrose) sweetens, but it has another role in ice cream: it changes the freezing point. When dissolved in water, sugar lowers the temperature at which water freezes. As more and more water freezes during the churning process (only pure water freezes), that sugar syrup gets more and more concentrated, further lowering the freezing point. (See more on this in Chapter 6.)
MILK SOLIDS NOT FAT
“Milk solids not fat” is an awkward term for everything in milk that isn’t water or fat: lactose, proteins, and salts. Lactose, a sugar, is 20 percent as sweet as sucrose (granulated sugar). While it doesn’t contribute much in terms of sweetness, it depresses the freezing point of the mix in exactly the same way as sucrose. The proteins in milk are integral to the structure and function of ice cream. Around 80 percent of milk proteins are casein, while about 15 percent are whey. Casein proteins form micelles, or organized groups. They are heat-stable but coagulate and clump together when exposed to acids (low pH)—as happens in cheese making. Casein proteins—which Roberts described in lecture as tiny tennis balls, or round masses with little hairlike protuberances around their exterior—act as an emulsifier when they coat fat globules and keep them separated.
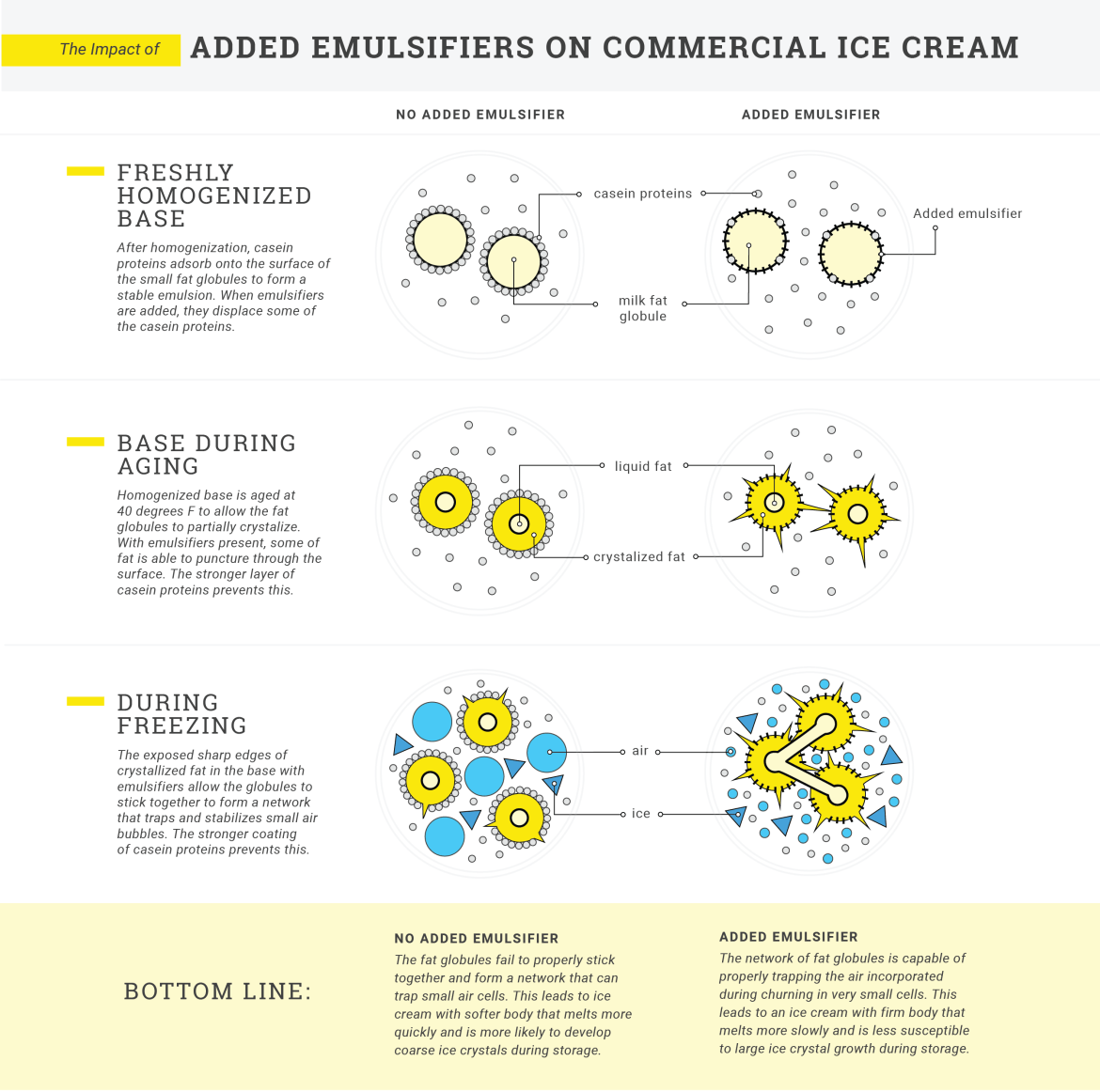
EMULSIFIERS AND STABILIZERS
Added emulsifiers and stabilizers are not present in all commercial ice cream bases, but they are in many. Emulsifiers in ice cream are seriously misunderstood. The truth is that when a mix is homogenized, the naturally present casein proteins (those little furry tennis balls) gather around the surface of the fat globules and keep them separated—and keep the mix emulsified. Commercial producers add emulsifiers like lecithin, mono- and diglycerides, or polysorbate 80 in order to dislodge and replace the casein. Why? Counterintuitive as it may sound, replacing the casein proteins with these added emulsifiers actually destabilizes the emulsification during freezing, allowing the fat globules to partially stick together once again, forming a network around air bubbles. If the casein proteins were left in place, they may prevent the fat globules from coming together in this way, making it more difficult to incorporate air and build a structure that resists melting.
Stabilizers, like carrageenan and various gums, help to maintain the small ice crystals formed through churning during hardening and storage. They accomplish this by binding water and increasing the viscosity of the mixture, helping to slow the rate of ice crystal growth.
Once these mix ingredients are combined (in different ratios, often calculated precisely by computer programs, or occasionally by hand), it’s time to pasteurize and homogenize. Only after all of this, does the ice cream base arrive at the scoop shop, ready to be made into ice cream.
06

Who’s at Ice Cream School?
During breaks in between lectures on ice cream, most short course attendees spent time discussing . . . you guessed it: ice cream. Over the years at America’s Test Kitchen, we felt like we had given a considerable amount of thought to all things ice cream, but we soon discovered just how intense the ice cream conversation could really get. Some of our more mundane discussions included: the pros and cons of accepting credit cards for orders under $5, how much to charge per scoop, how many quarts small store owners’ freezers can hold, and whether or not it’s possible to obtain and process raw milk from nearby farmers. Others focused on more specific challenges. One day we sat with the founder of a company that sold popular cupcakes. He was contemplating adding ice cream to their menu. Not on the side of the cupcakes, mind you, but actually in them. The consensus? Let’s just say we probably won’t be seeing soft serve–stuffed cupcakes on any of their menus in the near future. One classmate explained the travails of importing ice cream ingredients to Alaska. Another workshopped his dream of an ice cream sandwich store with anyone who would listen.
There was a robust group of attendees from large-scale ice cream companies. They were there to learn and to report back on what they learned to their employers. Not surprisingly, many from this group did very well in the short course’s final exam.
Many attendees were entrepreneurs who had either already opened small scoop shops, or wanted to. On the first day of the course, during the very long individual introduction exercise, Victoria Lai, whose email signoff includes the phrase “Icecreampreneur,” told the room that she had come to the short course for the first time in 2013. This was before she had launched her own ice cream business. Today she is founder and owner of Ice Cream Jubilee in Washington, D.C., which Tastemade recently named one of the 10 best ice cream shops in America.
We met Pichet Ong a few days in. He quickly became known as someone who knew where to get the best food in State College, Pennsylvania (not an easy task).
“I came [to the short course] because I want to understand manufacturing,” he told us. “To go from 3 quarts to large batches. I wanted to learn the language and the business of ice cream. Right now, I also have a fantasy to start my own ice cream company. Penn State is a good place to learn.”
He continued: “It’s a lot more science and less hands-on than what would work for me. But it’s all useful. It’s things I wouldn’t learn on the job. This class works well for people who are obsessed with process.”
07

The Process
Say you’re a scoop shop owner. You have your base mix. You’ve flavored it. Now you must age it. Aging the base involves simply allowing it to sit in the fridge for some time, often overnight. This process allows the fat droplets to partially crystallize so they are better able to stick together during churning, stabilizing the structure of the ice cream.
Next up? Freezing. While the act of freezing ice cream sounds easy, that’s not the case. The key to great commercial ice cream is closely tied to the freezing process: how fast it’s frozen, how much air is added during freezing, and how stable the ice cream stays after the initial freeze.
Freezing accomplishes two objectives. First is the rapid removal of heat that leads to the formation of small ice crystals. The goal of the commercial ice cream maker is to rapidly freeze their ice cream base. Why? Speedy freezing isn’t just good for production and the bottom line, it also makes a superior product because it leads to the formation of very small ice crystals and a smoother ice cream. Remember, the larger the ice crystals, the icier the mouthfeel. And icy is not delicious.
Second, freezing allows for the incorporation of air through the whipping action of the dasher blade, or the blade that scrapes the walls of the freezing surface and moves the ice cream mix in the machine as it freezes. The amount of air that is whipped into the base during freezing is referred to as overrun. It is calculated as the increase in volume over the original volume of the base. An ice cream base that doubles in volume during freezing has 100 percent overrun. Dense super-premium ice creams sport very low overrun, often around 20 percent. This number matters because the amount of overrun in an ice cream has an enormous impact on texture, flavor, melting qualities, and even perceived temperature—not to mention value. Fourteen ounces of base can be churned into a pint of super-premium ice cream, or nearly a full quart of the cheaper stuff. No successful ice cream entrepreneur forgets that when you’re selling ice cream, you’re also selling air.

Water has a single freezing point (32 degrees Fahrenheit or 0 degrees Celsius). When particles are dissolved in water, that freezing point goes down, meaning the water will need to get colder before it turns to ice. This phenomenon is referred to as freezing point depression. Interestingly, ice cream base doesn’t have one static freezing point, but rather a freezing curve. And here’s why: as pure water in the mix turns to ice crystals, it leaves behind a more concentrated solution of sugars, proteins, stabilizers, and salts that further decreases the freezing point. The upshot is that even when ice cream is fully hardened in the freezer, it still contains a highly concentrated solution of sugars and up to 10 percent liquid water. That’s why ice cream is scoopable while ice cubes are not.
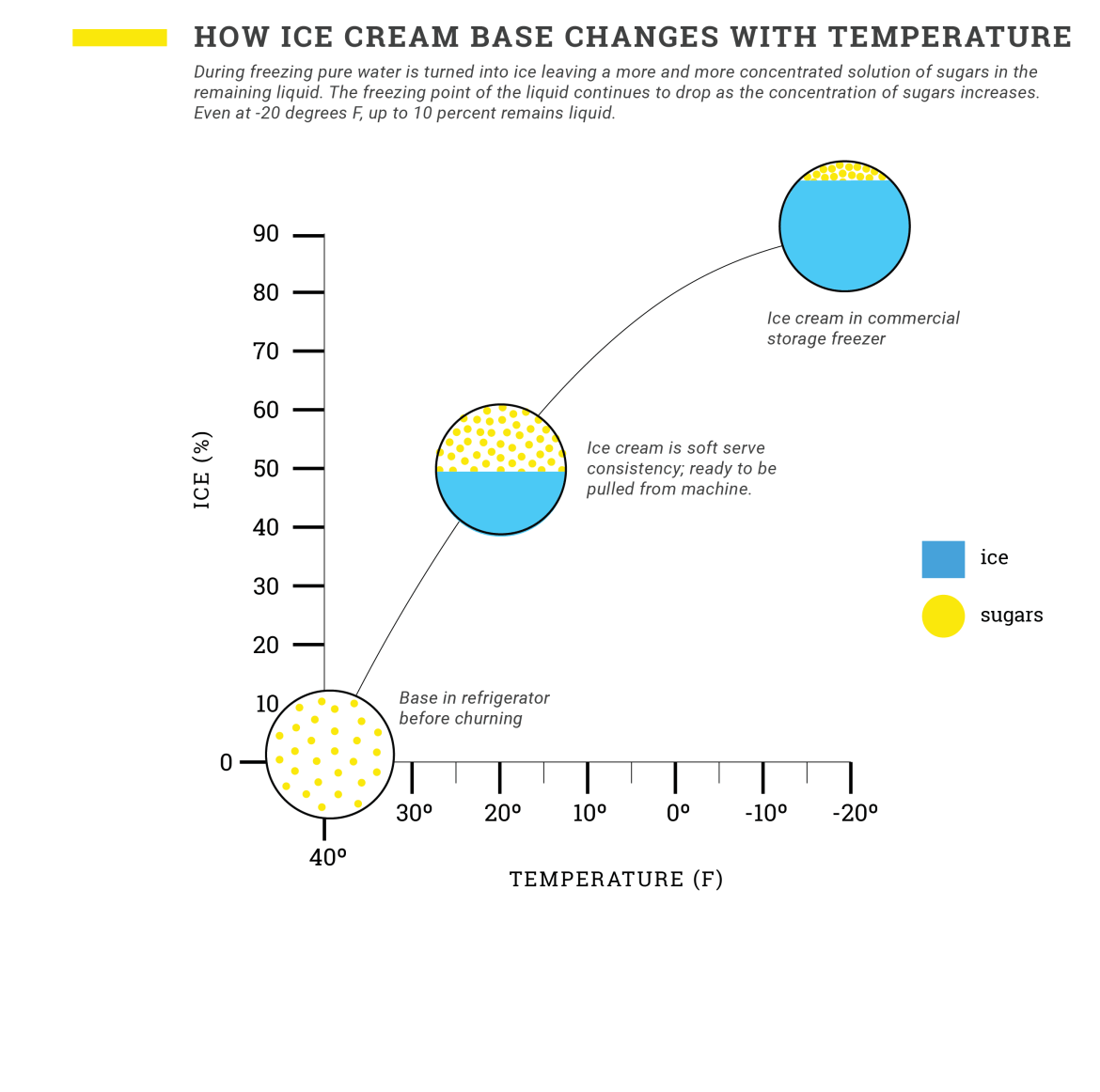
It’s important to freeze quickly, but it isn’t easy. In order to rapidly remove enough heat to drop a 40-degree Fahrenheit base down to about 21 degrees Fahrenheit and turn 50 percent of the water to ice, you need a powerful ice cream freezer—and that power comes with a big price tag and a large footprint.
There are two broad categories of ice cream freezers: batch and continuous.
When ice cream comes out of a batch or continuous freezer, only about 50 percent of the water has been frozen. And yet, it holds the maximum number of ice crystals it will ever contain. The remaining freezable water turns to ice during the time the ice cream is left to harden (the final step in this process) and doesn’t form new ice crystals, but rather increases the size of the ones already present. To keep ice crystals as small as possible, speed is still the name of the game. To that end, at large production facilities hardening takes place in the package, in huge walk-in freezers set to about -25 degrees Fahrenheit with high-velocity fan systems; scoop shops often employ smaller blast freezers for this purpose. The goal of this step is to cool the churned ice cream from its draw temperature (the temperature at which it exits the ice cream freezer) of 19 to 22 degrees Fahrenheit down to 0 degrees Fahrenheit in 4 to 7 hours. From there, the ice cream is transferred to a storage freezer set to -20 degrees Fahrenheit (sometimes as low as -30 degrees Fahrenheit). At this supercold temperature, the ice cream can be held without any noticeable reduction in quality for 6 months at a bare minimum. In one of our labs, we tasted excellent samples that were a full year old.
But when the ice cream leaves the production facility for distribution, that supercold temperature is no longer achievable. The ice cream is then much more susceptible to heat shock, where ice crystals melt and then refreeze into larger, coarser ones, which affects the ice cream’s texture. This is where stabilizers really earn their keep—slowing the movement of water molecules and preventing small crystals from growing into larger ones.
08

Back to the Test Kitchen
Because we spent most of our time at the Penn State Ice Cream Short Course in lectures and not actually eating ice cream, we were very excited to start churning our own ice cream when we returned to Boston. The goal? To develop a premium ice cream base (with great flavor variations) using the lessons we learned from the course.
We began by tracking down the commercial ice cream bases we’d heard so much about. We found a dairy purveyor in Chelsea, Massachusetts, just over the Mystic River from Boston, and purchased one 5-gallon sealed plastic bag (the smallest size and most convenient form they come in) of each of three different commercial bases—one 16 percent fat ultrahigh-temperature pasteurized base and one 14 percent base from Hood as well as a 14 percent fat mix from Week’s. We also obtained a sample base from Straus Family Creamery in Marshall, California, which is not for sale to consumers.
In the kitchen, we churned a quart of each mix in a Cuisinart ice cream maker, which uses a prefrozen canister and turns the base into the consistency of soft-serve ice cream in about 30 minutes. After hardening each sample in the freezer overnight, our team tasted them. The flavor was just what we expected—a clean, creamy blank slate. We also expected a supersmooth texture. After all, these ice cream bases had been perfectly calculated, mixed, homogenized, and pasteurized—they were engineered to make excellent ice cream. Yet, every one of them had noticeable (albeit mostly small) ice crystals.
Had we been lied to? Was commercial base really not all it was cracked up to be?
No, it turns out that the base wasn’t to blame, but our ice cream maker. Commercial batch ice cream machines go from liquid base to soft-serve ice cream in 6 to 8 minutes. That kind of superfast freezing creates tiny, imperceptible ice crystals. Churn that same base 5 times slower in a home machine and be prepared for some bigger, unpleasant ice crystals.
And that’s the hard truth—your home ice cream maker just isn’t very good. (Don’t feel bad, no one’s is.) It just simply isn’t powerful enough to remove heat rapidly and create small ice crystals. Beyond a lack of freezing power, the dasher blade is made of plastic (instead of sharpened metal) and doesn’t effectively scrape the wall of the canister. Instead of shearing off tiny ice crystals as soon as they form, it allows an insulating layer of hardened ice cream to form on the canister wall (which further slows freezing). The only ice crystals that are ever formed in an ice cream are the ones that are scraped from the sides of the ice cream machine. After they drift into the middle of the ice cream base, they can only grow larger.
So what did we do? We realized that if we couldn’t fix the ice cream maker, we needed to fix the base. Some of the most successful home recipes that we’ve tried are Jeni Britton Bauer’s from her book, Jeni’s Splendid Ice Cream’s At Home (2011). Her base recipe calls for boiling the mixture to both reduce water and improve the protein’s ability to bind up water and prevent it from freezing. She also adds cream cheese to further increase the protein. The result of her technique is a very dense, creamy ice cream, but one with a cooked, slightly savory flavor. We wanted a similarly smooth, creamy texture but with the blank slate flavor we liked in the commercial bases. So Dan spent a month in the kitchen testing mix compositions. (Molly tasted with enthusiasm.) He took what would be a standard formula for a premium ice cream base with 14 percent fat, and then started to break the rules. As fat content goes up, commercial producers generally lower the amount of milk solids not fat (MSNF) to keep total solids at about 40 percent. Dan did the opposite, increasing MSNF by adding nonfat dry milk powder until the total solids hit about 46 percent. Not only was there less water in the mix proportionally (so less potential for turning into ice), but milk powder provides additional benefits. The protein in milk powder hydrates during heating, binding up water that would otherwise turn to ice. Milk powder is also rich in lactose, which depresses the freezing point without adding much sweetness. Increasing lactose meant we could bring down sucrose and make an ice cream with the right texture but that wasn’t too sweet.
Two final additions, which are also in Jeni’s recipes, helped us in our quest for smooth ice cream—corn syrup and cornstarch. Corn syrup is a mixture of glucose molecules and broken starch chains—it adds viscosity to the mix, slowing the movement of water molecules and keeping ice crystals from growing larger and coarser during hardening and storage. Readily available cornstarch binds water when we heat our base up to a simmer, decreasing the amount of freezable water in the mix. Cornstarch also acts as a stabilizer, helping to keep ice crystals small during hardening and storage.
With a rock-solid (but very scoopable!) base on our hands, we finally got down the fun of making flavor variations. After a round of voting with the team on favorite flavors, we settled on four: white coffee chip, strawberry ripple, peanut butter cup crunch, and vanilla bean.
09

Finale
On Wednesday, Thursday, and Friday afternoons of the short course, we left the fluorescent confines of the conference room to attend lab sessions on the Penn State campus. There were labs on sensory evaluation, freezing, mix calculations, and more. On Thursday, we attended the Vanilla Lab, where we were led by Skip Rosskam, president and COO at David Michael, a flavor company, through a smell and taste exercise with samples of vanilla beans from Mexico, Madagascar, and Tahiti.
“Sniff each one,” said Rosskam. “What do you smell?”
Words were thrown out: cherry, cardamom, Christmas. The scent changed significantly when we began smelling a different vanilla bean, the Madagascar, versus the Bourbon bean. Our small group then tried our hand at combining solutions (containing 35 percent alcohol) of each variety in order to make the ultimate vanilla flavoring. Rosskam would bring it back to David Michael, he said, and a tasting panel would compare our vanilla flavor in an ice cream to the vanilla flavors made by other groups from the short course. The winners would get a prize.
As soon as we finished in the Vanilla Lab, we loaded onto a bus and were driven to the Penn State Dairy Barns, where we took a tour of the grounds, wending through earthy-smelling barns and silos. We visited the calf barn and, not surprisingly, oohed and aahed over the wobbly, eager calves. We visited the feed center, where we learned about the cows’ carefully regulated diet as we listened to the sound of slow, methodical chewing. In the Intensive Research Barn, we watched our tour guide reach into a cow’s rumen, through an implanted rubber hole, and pull out some partially digested food. We paused in the area where cows were being mechanically milked and then stopped in front of the tank where the minutes-old fresh milk was being stored. It would soon be pasteurized and shipped off to places like the Penn State Berkey Creamery, where it would be joined by some combination of cream, sugars, stabilizers, and flavorings. The room was humid, the tank was humming, the air smelled like a mixture of manure and machinery. We could hear the sounds of cows braying in the distance. This was the beginning.
Field photography by Dan Souza.
Test Kitchen photography by Daniel van Ackere and Joe Keller.
Food Styling by Elle Simone and Catrine Kelty.
10

Recipes
We’ve put a lot of thought into how we present our recipes at Cook’s Science and we are excited to share some key features with you. First, we’ve provided ingredients in both grams and traditional US volume and weight measurements so you can work with whichever you prefer. You can toggle back and forth by clicking GRAMS or TRADITIONAL at the top of the ingredient list. We’ve never loved jumping back and forth between the ingredient list and procedure while we’re busy cooking so we’ve repeated the ingredients above the step where you need them. And finally, we’ve built in a pretty slick annotation feature to provide key information, photographs, and video throughout the recipe, without getting in the way of the clean, simple recipe view. Click on any of the yellow highlighted words to access the annotations. We hope you’ll check it all out and let us know what you think.

