Fizz Ed

01
Packing a Punch
In a low beige building in eastern New Jersey, specimen cups full of brilliant green liquid line a laboratory counter. I sip the first. Candy-sweet, moderately acidic, with a complex, juicy flavor of hard-to-identify fruit. Strawberry? The neighboring cup, in a somewhat paler shade of grass green, is just as sweet, but the fruitiness tastes less . . . round? Less full? Sweet in a different way? I don’t quite have the vocabulary to pinpoint the difference, but Elaine Kellman-Grosinger does.
“The berry is right, but it needs to be a little punchier,” she says after sampling the beverages, all of them flavored blends containing 5 percent juice, which she’s creating for an unnamed customer. “Punchier” as in the flavor of fruit punch, which, she explains, is a surprisingly complex hybrid made up of a handful of different flavors, including pineapple, cherry, and citrus.
After getting her master’s degree in organic chemistry, Kellman-Grosinger worked at some of the flavor industry’s multibillion-dollar companies: Givaudan, Symrise, International Flavors and Fragrances. In 2005, she was hired by Citromax, at the time just a citrus extract company, to create a new flavor development division. A new floor—including the lab we’re in—was added to the building to house Citromax’s thriving flavor business.
I am behind the fragrant scenes of the often-secretive flavoring industry—the world of companies that create the flavors of much of what we eat and drink. Sodas, ice creams, frozen dinners—all of these contain chemical flavorings designed in labs like this one, though many makers would rather not call attention to that fact. A room lined with bottles on floor-to-ceiling shelves contains hundreds of flavors that Citromax has created for its many clients, neatly alphabetized: cappuccino, cantaloupe, salted caramel.
When most of us laypeople taste berry flavor, we can identify that it’s berryish, and maybe a couple of additional details: how sweet or tart it is, if it leans toward raspberry or strawberry. A trained flavor chemist like Kellman-Grosinger, however, is capable of pulling out dozens of descriptive words to characterize just what sort of berry flavor it is: floral, jammy, fresh, musty, sulfuraceous. A requisite for the job, she says, is a “subthreshold” sense of taste and smell—the ability to perceive flavors more subtle than most people can notice. It’s an ability that’s partly innate and partly learned.
I follow her back to her office, which adjoins the lab. At a computer, she pulls up the formula for the flavor of the second drink. It’s a recipe containing some 40 fundamental ingredients—hexyl acetate, damascenone, distilled lime oil—in milligram quantities, all of which add up to what we’ve been sipping. With practiced speed, she tweaks a few of the abstruse ingredients on the screen to bring up the punchy flavor of the drink: a little more allyl caproate, a little more benzaldehyde, a pinch of furaneol.
02

Creating Flavor
The history of soda flavoring begins in the pharmacies of the 19th century, where carbonated drinks were originally considered health beverages but soon were drunk for pleasure. Pharmacists were among the first flavor chemists.
According to Nadia Berenstein, a flavor historian at the University of Pennsylvania, “Pharmacists not only made medicines, but they would also do minor chemical work, which included manufacturing sparkling water. They made different kinds of coloring and flavoring products for their medicines, because medicine was bitter and unpalatable, and then also for their sodas.”
And those flavors were compounded of more than just fresh fruits and spices. By the middle of the 19th century, it was widely known by pharmacists and confectioners that certain simple synthetic chemicals had distinct fruity flavors. These chemicals found wide use as an inexpensive, easy-to-manufacture means of providing consistent, intense, and novel flavors to candies and beverages. The 1897 edition of Emil Hiss’s Standard Manual of Soda and Other Beverages gives guidelines for the soda fountain proprietor. It contains dozens of recipes like the one for Raspberry Essence or Extract, which calls for nitrous ether, acetic aldehyde, formic ether, butyric ether, benzoic ether, oenanthic ether, sebacic ether, acetic ether, oil of wintergreen, amyl acetate, amyl butyrate, saturated alcoholic solution of tartaric acid, and saturated alcoholic solution of succinic acid.
(Show that ingredient list to the next person who tells you he avoids synthetic ingredients because his great-grandmother wouldn’t have eaten them. My own great-grandparents met over a raspberry soda.)
In the 20th century, improved bottling procedures allowed particular flavor combinations to spread beyond the local soda fountain and go national. Many of these were based on a boom of newly synthesized ingredients, like methyl anthranilate, which was first used in the perfume industry as an inexpensive substitute for orange blossom oil, until soda makers realized that, at a higher concentration, it had a grape-like flavor. Methyl anthranilate came to be synonymous with grape flavor, as the basis of numerous popular purple sodas created starting in the 1910s, including NuGrape, Grapico, and Grapette—all of which are still drunk today.
In her doctoral dissertation, Berenstein writes that methyl anthranilate became grape flavor in the popular consciousness “by its repeated and continued use in grape flavorings, in alliance with other substances, such as tartaric acid, sugar, and, notably, purple coloring, that were made to signify and reinforce the sensation of grape-ness.”
“Some of the first flavor chemistry research was done at the behest of soda bottling associations,” says Berenstein. “Soda makers’ claim to the consumer’s palate was based on getting you to become loyal to one flavor and to want it over and over again.”
To that end, new flavor ingredients were developed and new flavors introduced. Some of these were intended to mimic familiar flavors—or even improve upon them. But others were based less on a real-world taste than on a creative notion, like pink bubble gum flavor or Kellman-Grosinger’s fruit punch. In the industry, these are called fantasy flavors.
In the United States, says Berenstein, “the popularity of imitation banana flavor predated the widespread consumption of banana by at least a decade.” And while the isoamyl acetate of banana flavor was ultimately detected in the fruit itself—well after the chemical was first synthesized—the flavor basis of strawberry candy, called ethyl phenylglycidate, is pure fantasy. “That compound has never been found in actual strawberries!” In attempts to regulate synthetic flavorings, government regulators developed techniques of chemical analysis—techniques later adopted by the flavor industry itself—to compare the chemicals in popular sodas with those in the fruits they claimed to taste like.
In 1909, a group of flavor companies formed the industry group Flavor and Extract Manufacturers Association (FEMA), which currently maintains a database of the almost 3,000 flavor ingredients that are “generally recognized as safe” (GRAS) by the U.S. Food and Drug Administration (FDA). Using that plethora of compounds, today’s flavorists are able to engineer flavors with dozens of nuances, which correspond more closely to our experience of a real fruit than the one-note grape or banana compounds of 100 years ago. Alternatively, they’re also able to concoct elaborate fantasies that evoke “not existing foods, but states of mind or states of being,” Berenstein says, like Gatorade No Excuses.
03

Designing a Drink
Today, every soda on the shelf is a carefully engineered product of the flavorist’s art, its nuances of refreshment and stimulation fine-tuned to make it as craveable as possible in an enormously competitive market. Soft drinks in the United States are a more than $100 billion industry, according to the data clearinghouse Statista.
Does it need a little more sweetness, a fuller-bodied mouthfeel, a hint of the astringent taste that signifies “energy drink” to the palate? All of those are added by experts like Kellman-Grosinger.
Say you’ve figured out a wonderful recipe for a ginger-kiwi soda, and you’re ready to go commercial with it. You contract with a manufacturer to produce it in bulk and bottle it, but grating fresh ginger and juicing fresh kiwis—let alone acquiring them from a consistent source—becomes a daunting challenge when dealing with hundreds or thousands of pounds per batch. You need to create a recipe that scales up. Time to call a flavor company. Companies like Citromax and Louisville beverage development house Flavorman work with clients of all sizes, from the largest multinational beverage brands to home-kitchen entrepreneurs.
Professional flavorists like Kellman-Grosinger and Ted Roszel, who is a “beverage architect” at Flavorman, have naturally keen senses, improved by training and years of experience tasting, analyzing, and understanding what makes flavors tick. Their jobs involve being able to replicate a flavor—whether in nature, in a small-batch recipe, or, quite commonly, making a knock-off version of a successful drink—using chemical formulas.
I gave Roszel a hypothetical curveball: how would he make a rye bread–flavored soda?
“First off, we would eat rye bread and take notes,” he said. “You’ll taste some spiciness from the rye grain, you’ll taste caraway seeds, probably some yeastiness.” With that as a starting point, he’d start drawing up a list of chemical ingredients he might put in the soda formula.
“We’d want a minty wintergreen note for the caraway. Probably some esters come through from the yeast. There’s also got to be some diacetyl, for a buttery note. And there are some sugar reactions that create that sort of molasses note, if it’s a dark rye.”
But these flavor notes, mainly perceived via the sense of smell, aren’t the only element to creating a successful drink. Because flavor, when it comes down to it, is a combination of all of our senses—smell, taste, touch, sight, and sound—coming together to create a full picture in the brain.

In terms of texture, added ingredients can make a beverage fuller- or lighter-bodied, and affect how it lingers on the palate, but, once again, most of it is in your mind. “If you’re making a chocolate brownie–flavored vodka—or a bread-flavored soda—the sensory experience is very different between drinking a beverage and eating a chewy brownie. But if you label your vodka ‘Chewy Brownie’ flavor, that goes a long way to plant in your brain what we want you to think when you’re drinking this.”
The visual impact of a soda contributes to the experience of its flavor more than we realize, so that too is tweaked until it’s optimal. In a famous marketing experiment conducted in 1957, when 7-Up soda cans were colored with 15 percent more yellow, without changing the content, consumers agreed that the soda tasted more lemony than before. And, in 2014, when wine experts (as well as amateurs) tasted the same wine under different colored lighting, they described it very differently.
“A lot of your impression of flavor is actually the color,” says Flavorman’s Roszel. “The orange soda you loved as a kid—it’s just sugar, water, acid, and maybe a tiny bit of citrus flavor, but it’s that bright orange color that counts.”
Beverage makers can even add a clouding agent to an otherwise clear drink, to give it the hazy appearance of juice. At Citromax, tastings of new formulations are done in special isolated booths where the lighting can be modified to obscure the color of whatever’s being tasted, so as not to skew the results.
04

All Natural
For an ingredient to be cited as “natural flavor” on a label, the FDA requires that the original raw material for the ingredient must come from an animal or plant. That definition includes lemon oil distilled from lemons; but it can also include vanilla flavoring that’s manufactured from rice bran and has never been anywhere near a vanilla bean, since rice bran is a plant product.
Conversely, an “artificial flavor” is any flavor that’s not derived from one of those sources—although it can be exactly the same chemical, just created in a different way. For instance, the same vanillin molecule—C₈H₈O₃—can be extracted from cured vanilla beans, derived from rice bran, or synthesized from petrochemicals.
Today, flavor labs use both natural and artificial flavors for their creations, depending on their final destination. It’s a complicated chemical dance, one that’s rarely acknowledged by the average commercial beverage drinker. “When somebody looks at the ingredients list on the back of the bottle, and it says natural flavors—that’s us,” says Lisa Deprospero, a food technologist at Citromax. “Most people don’t realize that everything they taste is made up of chemicals. Most people don’t even think about it.”
Of course, “natural flavoring” could have a different definition altogether. A comparable flavor to Citromax’s in-the-works punch beverage (though perhaps without the neon color) could be achieved by simply mixing fruit juices. But chemical formulas have a few advantages.
Pure juices are extremely variable, in quality and in price. To create a cocktail out of juices that will be consistent on a commercial level is a challenge. If one crop of fruit comes in significantly less sweet than the last batch, the whole recipe has to be adjusted to compensate. And, adds Citromax’s Deprospero, who’s responsible for assembling the batches of punchy beverages we’re tasting in the lab, fruit juices continue to change after they’re packaged, so any given bottle of real juice punch will taste different depending on how many weeks it’s been on the shelf. Added flavorings compensate for these issues; indeed, the “100% Pure Squeezed Orange Juice, Not From Concentrate” that you buy in cartons is often doctored with added “flavor packs” to give it a specific, consistent flavor.
But not everyone feels comfortable with the added chemical ingredients. Spindrift, a Boston-based maker of flavored sparkling waters, very proudly touts that, as of 2017, all its drinks are made of water, juice, and nothing else.
“At one point,” says Spindrift founder Bill Creelman, “a few of our products had what was called natural flavoring. We did what everyone else does: We paid a flavor house, and we would ask them for a natural flavor that we’d add in with the juice. They might send five different choices, and we’d choose the one we thought was the best fit.
“But if you ask what is in the natural flavor you’re buying, they’re not required to tell you. And it may be completely acceptable and aboveboard, but I couldn’t get comfortable.”
(Citromax’s Kellman-Grosinger says, “We do not divulge formulas. We can say that it contains a certain percentage of essential oils or essences, and a certain percentage of natural aroma chemicals that are all approved by the FDA and all GRAS.”)
As Spindrift reformulated its products over the last few years to omit the added flavorings, there were new challenges, he says.
“We had to add more juice—and fresher juice. We need to make sure we have relationships with the farms, that we’re shipping, receiving, and processing the ingredients on the right schedule. It’s definitely more expensive, but we feel it’s a better product now.”
He continues: “We have to process fruit in a cold environment, and we’re constantly managing variables like pulp and color, in the way that you would if you were cooking a meal. A synthetic flavor doesn’t have any microbiological properties. It has no color, you don’t have to pasteurize it, you don’t have to worry about its stability. You just dump it in the tank.”
And, appealing as the idea of pure juice is, the taste can be a harder sell. “Actual juice may be unlike what a customer’s used to, and that’s quite a challenge. Watermelon is one that we no longer make. I would say that Jolly Rancher has ruined the marketplace for fresh watermelon. People’s expectation of the taste of watermelon is not what you get when you actually make the drink.”
Portland Soda Works in Portland, Oregon, develops and manufactures small-batch soda syrups, using recognizable whole botanical ingredients, and deals with some of the same challenges.
Founder Chris Onstad started with root beer. “I went on the Internet and I typed in ‘root beer recipe.’ There are dozens and dozens. I went to my local herb shop. I went sniffing through all those jars of spices, and I chose which ones smelled like they’d work for me.” He crushes and toasts the spices to tease out the oils, boils them in a brew bag, like you would for tea, and then adds cane sugar and molasses as sweeteners. “Then vanilla, because root beer needs to have that creamy taste.”
But with whole spices, the nature of the soda changes over time in exactly the way that Citromax carefully engineers theirs not to. “In a brand-new batch, you can tell the cloves from the star anise, you can tell the molasses,” Onstad says. “After a couple of years, it smooths out; it’s much more homogeneous. It’s delicious, but fresh is best.”
05

A Little Sparkle
There’s one more element to take into account when creating a flavor for a soda: The carbonation that’s added—at the bottling plant, or when you mix one of Portland Soda Works’ syrups into seltzer at home—plays an important role.
The bubbles don’t just create a tingly sensation on your tongue. They have their own effect on the flavor of the soda. When a drink is poured, the effervescing bubbles bring some of the volatile flavor out of the soda and into the air, and continue to do so as you drink it, intensifying the retronasal flavor beyond that of a still drink. Carbonation also carries its own flavor too. When carbon dioxide dissolves in water, a modest amount of carbonic acid is formed, which gives the water a tart taste. If you taste seltzer that’s gone completely flat, it still has that weak sourness.
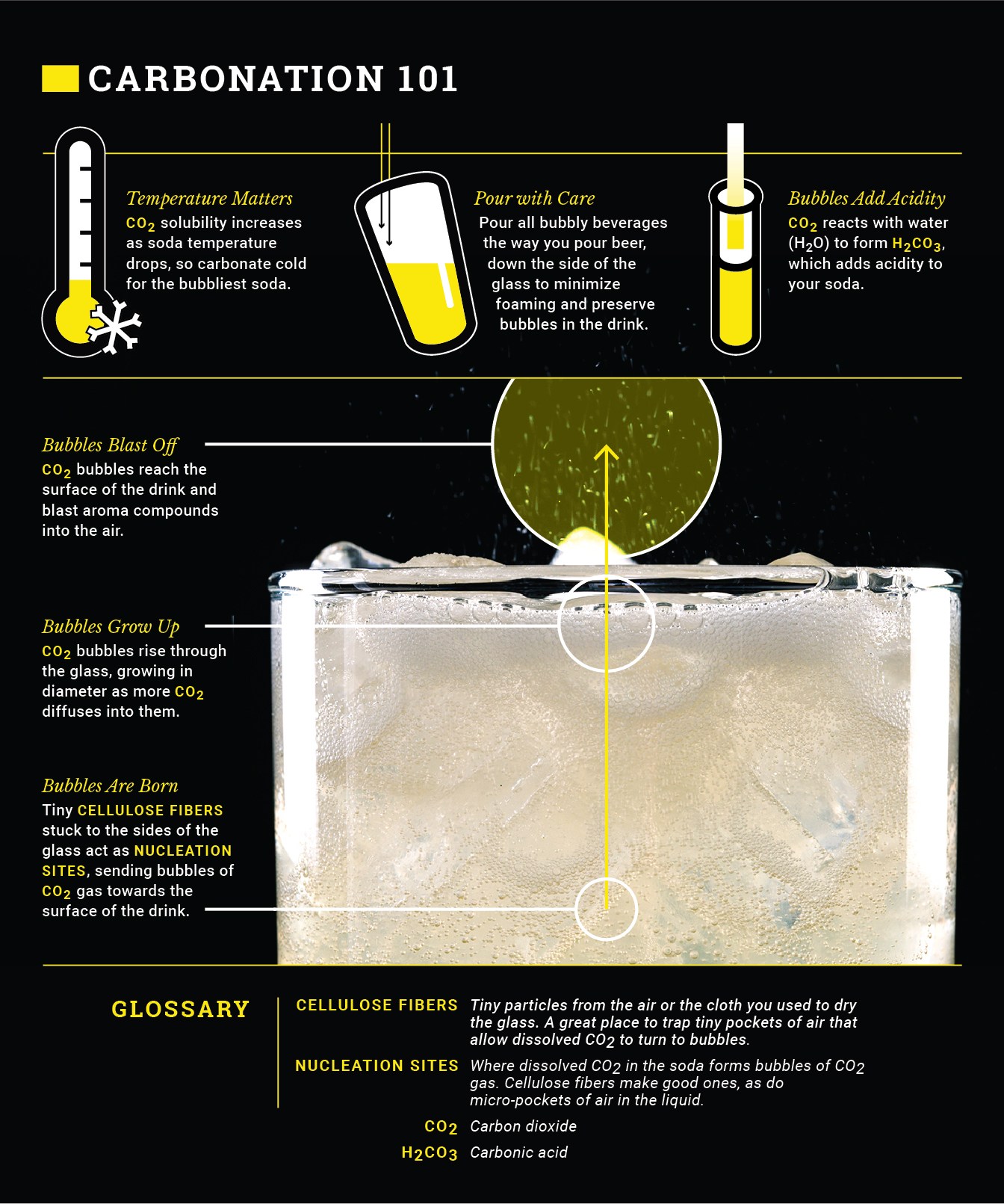
However, a groundbreaking study published in Science in 2009 established that most of the taste of carbonated water is in fact created when you drink it. As the liquid passes over your tongue, a fast-acting enzyme, activated by the carbon dioxide, frees some positively charged protons from the drink, which trigger your sour taste receptors, giving soda its distinctive tang and tingle. That must be accounted for by soda flavorists.
For Spindrift’s Creelman, “the more carbonation you have, the more flavor. But higher carbonation starts to distort the flavor and takes away from the subtlety of the ingredients.”
Portland Soda Works’ Onstad prototypes all his syrups with carbonated water. “Carbonation adds acidity to soda, and every syrup flavor has to take that change into account. The entire character of a drink can shift when it’s carbonated. “After a night of ‘dwelling’ [under carbonation], some ingredients are muted while others are amplified. I don’t have a good rule to make the results of acidulation more predictable, unfortunately, because the chemistry at hand is so complex.” Neither does Citromax’s Kellman-Grosinger: “There’s no rule of thumb. Sometimes the heavier flavor notes get masked by the carbonation, so you need to add more of them. Sometimes you don’t change anything.”
“Our national palate is very used to acid, because so many packaged foods are preserved with it,” says Onstad. “To read as familiar, as delicious, as actual beverages in the way that people are imprinted from childhood, they need that acid.” Carbonation provides some of it but additional acid flavorings are typically added to the mix. “We use different acids, depending on the ingredients: citric, malic, or tartaric. Citric amplifies fruit flavors. Malic is like turbo-citric, much more tart. Spices like clove, cinnamon, nutmeg, and sarsaparilla taste terrible with citric acid, so . . . if we’re working with brown flavors or spices—in the coffee, in the root beer—we use ascorbic acid, which is much milder, and let the carbonation” do the rest.
06

Dialing It In
Kellman-Grosinger’s new formula for the green fruit drink prints out in the lab, where technician Michael Filardo starts hurriedly compounding it in a beaker, dipping disposable micropipette dispensers into brown glass vials pulled from a shelf of hundreds, and weighing out each precise drop. I take the opportunity to cautiously wave some of the bottles under my nose. Benzaldehyde has the sweet smell of almond extract or cherry pits; allyl caproate smells like a million pineapples concentrated into an ounce of viscous fluid. So this is where that refreshing fruit punch of my childhood came from.
The last milligram of gamma-decalactone is added to the new, punchier version of the green fruit formula, and the concoction gets stirred for several minutes on a magnetic stir plate. The final result is a few grams of clear liquid in a glass carafe. In its pure form, it smells a little bit like fruit and a little bit like airplane glue. Deprospero measures and mixes two-tenths of a gram of it into a full cup of the bright green neutral-flavored 5-percent-juice base, and we pour out fresh samples.
With a fuller backdrop of fruit-punch flavor, the new version is a lot closer to the target. It still needs a tweak to the acidity level, we agree, and a little more ripeness in the berry, but it’s just about ready to present to the client. Next up: a bright blue drink.
07

Back to the Test Kitchen
[Ed note: As senior editor Paul Adams reported and wrote his soda story, executive editor Dan Souza began developing his own recipes for the home cook. The following is from his perspective.]
Most of the time when I’m thinking deeply about beverages there’s alcohol involved. When I decided to develop a couple of soda recipes to accompany Paul’s lovely deep dive into flavored beverages and bubbles, I was worried I might find soft drinks a bit . . . sober. Among its other dazzling properties, alcohol is a great carrier of flavor, which is why boozy drinks can taste so delightfully intense. But when I removed alcohol from the equation, I found myself focusing more deeply on other, surprising elements of flavor. And when it comes to soda, flavor is everything.
I set my sights on two combinations: lemon-lime and strawberry-rhubarb. Step one: Create a flavorful base using (mostly) fresh ingredients. (No allyl caproate here.) Step two: play with carbonation.
Double Lemon-Lime
Darcy O’Neil, chemist turned bartender turned soda expert, explains in his book Fix the Pumps that the first popular soda fountain flavor was lemon. Lemonade was a fashionable beverage around the turn of the 19th century, so it stands to reason that a carbonated version of it would be equally a la mode. But O’Neil explains that that wasn’t the whole story. Lemon was popular with soda jerks (who operated the soda fountains) and pharmacists (the original soda makers) in a large part because lemon peel is saturated with flavor-packed oils, which have a long shelf life once extracted (citrus juice, on the other hand, deteriorates rapidly and, thus, has no place in syrup). A strawberry tastes great, but it has no oily peel, so its flavor is all in a watery form that’s only good when very fresh.
I wanted to riff on the original fashionable soda flavor, as well as one of most enduring bottled sodas of all time (I’m looking at you, Sprite) by incorporating lime and then adding some welcome intensity with a couple of secret ingredients. I chose to take my lemon-lime soda into double lemon-lime territory (!) by adding makrut (also known as kaffir) lime leaves and lemon grass. Fresh makrut lime leaves (which are easy to buy online in small quantities) have a resinous, concentrated lime aroma that serves them well in curries, soups, and stews in Southeast Asian cuisines. And accurately named lemon grass is a grass with a lemony aroma, also popular in tropical parts of Asia.
To make a concentrated syrup, I combined lemon and lime zest, lemon grass stalk, and lime leaves with water and sugar, brought it to just a simmer on the stove, turned off the heat, and let it steep, covered. I wanted enough heat to speed extraction but not so much that would damage delicate volatile aromas, or drive them into the air and out of the syrup. After straining, I was left with an intensely heady, lemon-lime syrup. And, while it made for a lovely, aromatic soda when I added soda water to the mix, it lacked the citrusy acidity I wanted. This soda needed a bit of a pucker. I solved the problem by adding a dry acid that I ordered (easily!) online, one that you often see listed on the back of a can of soda (for good reason, it turns out): citric acid. Citric acid added sharpness and made the soda taste more like lemon and lime.
Fresh Strawberry-Rhubarb
There may be no flavor combination more near and dear to my heart than strawberry and rhubarb. I come from a family of Mainers and pie makers and a childhood home with a rhubarb plant the size of a Volkswagen Beetle. But I’ve never had a strawberry-rhubarb soda. And I really wanted one. I love the combination of jammy, stewed strawberries and rhubarb in pie filling. But when it came to adapting the pair for soda, I knew I wanted fresh, not cooked, flavor.
Unlike citrus peels, lime leaves, and lemon grass, strawberries and rhubarb aren’t loaded with flavor-packed oils that can be extracted into a concentrated syrup through gentle heat. To get the experience of eating a fresh strawberry combined with the brilliant acidity and astringency of raw rhubarb I would need to carbonate their uncooked juice. Rhubarb is easy to juice and I had quick success blending or food processing it into a puree and then straining it through cheesecloth. But try blending and straining strawberries to get their juice and you end up with a pulpy, gelatinous mass that simply refuses to give up its liquid. If you have a centrifuge, you can go the route of the guys over at ChefSteps and rapidly separate out the juice from the pulp. I don’t have a centrifuge (I know I’m not alone here), so I wanted a lower-tech solution to the problem. I reached out to someone I figured may have already come up with it: Evan Harrison, part owner and bar manager at Mamaleh’s Delicatessen in Cambridge, Massachusetts.
Harrison’s background is behind the bar, but at Mamaleh’s, he’s stepped up to the fountain, so to speak, and created 13 house-made sodas. My favorite is his pineapple lactart (lactart is a natural milk-derived acid that adds a pleasant sourness to sodas, and was marketed for this purpose in the 1880s). The soda is crystal clear with the faintest yellow hue, but it’s absolutely bursting with intense fresh pineapple flavor. I called him up and asked him how he does it. Harrison explained that he combines fresh pineapple with sugar, seals it in a vacuum bag, and stores it in the refrigerator for up to 6 days. Then he muddles the mixture by hand before straining off the syrup. In the kitchen we call that maceration, but it’s usually done on a much shorter time scale—letting the fruit and sugar sit together for only 30 to 60 minutes—and with the goal of pulling excess liquid from the fruit, not infusing the fruit flavor into the liquid. But it made sense. Infusion doesn’t only happen when heat is applied, it just happens faster with heat. (Think about the brewing time difference for cold-brewed and hot-brewed coffee.) Leaving the exuded juice in contact with the fruit for an extended period of time allowed it to pick up lots of fresh flavor.
I tried it with my strawberries. I tossed a pound of sliced berries with some sugar, popped them in a zipper-lock bag, and let them macerate for different lengths of time, from 30 minutes (at room temperature) to up to 4 days (in the fridge). The same-day batches had weak flavor, but after 12 hours the syrups were delicious. All I had to do then was strain the syrup through cheesecloth (and reserve the nice macerated berries for topping yogurt or oatmeal), combine it with my rhubarb juice, and carbonate. There was no need for lactart (or citric, malic, or tartaric acid, for that matter) because rhubarb is packed with its own bright acidity.
Carbonation
As a kid I used to hate carbonated beverages and spicy foods. I hated them for the same reason: They hurt my mouth. Although they hurt in different ways, it turns out that both types of food exert their painful properties in the same way—certain molecules (carbon dioxide and capsaicin, respectively) each trigger pain receptors on the tongue. I grew out of my dislike, however. These days I love and crave both bubbles and spice. And to be honest, over the last few years I’ve gone carbonation crazy, investing in the CO₂ tank-regulator-and-carbonator cap setup that Dave Arnold introduced me to in his seminal Liquid Intelligence. This allows me to carbonate pretty much any drink I want, at any time I want—no added club soda needed, which means no dilution. I can rest easy at night knowing that I am always minutes away from carbonated cocktails, soda, grapes, tea, you name it.
Making my Double Lemon-Lime syrup into a soda couldn’t be simpler: pour the concentrated syrup into cold club soda (or homemade seltzer) in a ratio of 1 part syrup to 4 parts soda water (give or take, depending on preference). It works because the syrup is concentrated enough that the finished drink is plenty bubbly and plenty flavorful. But that won’t fly for the strawberry-rhubarb soda, since it’s made with uncooked juice rather than syrup—if you dilute the juice at a 4-to-1 ratio like the syrup, you’d end up with a seriously diluted drink. The answer? Force carbonate the whole dang thing. If you don’t, understandably, have Arnold’s CO₂ tank-regulator-and-carbonator cap setup, your next best bet is using a cream whipper. You may be tempted to use your SodaStream to carbonate this soda, but please don’t do it. Not only will doing so void the machine’s warranty, it will also likely ruin it as sugary soda flows from the bottle up into the gasket. (Check out the recipe to learn the exact steps.)
What happens when you put juice in a cream whipper (or water in your home seltzer maker) and charge it with CO₂ gas? You are pressurizing the container, and by doing this, you are forcing CO₂ gas to dissolve into the liquid (hence the term force carbonation), where it will stay so long as the container remains under pressure. In that closed system, there is equilibrium—the amount of CO₂ gas dissolved is dependent upon the pressure in the container. Once the container is opened, that pressure is gone, causing dissolved CO₂ in the liquid to turn back into gas and exit. As a home carbonator (and consumer of carbonated beverages), you have a lot of control over how all of this goes down.
When force carbonating, you want the juice (or water) to be to as cold as possible without being frozen. CO₂ is more soluble in colder liquid, so if the liquid is just above freezing, it will dissolve maximum CO₂, and then be very bubbly when you drink it. The same rule applies at serving time—keep the drink cold and you will keep more CO₂ in the drink and have less of it exploding out the top of the bottle.
For the best possible carbonation, you also want your liquid to be clear and free of stuff. Stuff includes everything from air to fruit pulp to small fibers deposited by the towel you used to dry the container the juice is in. Why? In the same way that Clark Kent needs a phone booth to get on his Superman tights before he can save the world, CO₂ that’s dissolved in liquid needs a place to change back into a gas bubble. And that place is called a nucleation site. Nucleation sites are found in cellulose fibers from your drying towel, suspended particles, or existing air bubbles. If you have too many nucleation sites when carbonating (you can’t get rid of them all within the liquid), you’ll experience lots of frothing, which means a lot of CO₂ is coming out of solution, so when you drink the beverage, it’s less-than-perfectly bubbly. You can go to great lengths to achieve perfect clarity—including adding pectin-destroying enzymes, centrifuging, and clarifying with agar or gelatin—but I don’t go too crazy for my strawberry-rhubarb soda. After juicing the rhubarb, I let it sit in the fridge while the strawberries macerate so that some solids can fall to the bottom and be left behind. Carbonation purists may wince, but when it comes to a juice-based soda, I actually like the creamy appearance and champagne-like head I get without clarifying. And I’m willing to sacrifice a little bubbliness for that look and the convenience of not clarifying.
It’s not at all hard to make your own soda out of any flavors you choose. Syrups are easiest to make and to work with, and they last in the fridge for weeks, so you can mix them into a soda (or cocktail) whenever you want. For citrus flavors, spices, and anything that doesn’t lose flavor when you simmer it, making a syrup is the way to go.
However, when you want to capture a really fresh fruit flavor, like Spindrift does, a better approach is to juice the fruit, add sugar and acidity to give it as much bright kick as you want, and then force carbonate it.
We can’t wait to hear what flavors you make!
Field Photography by Kevin White.
Test Kitchen and Styled Food Photography by Steve Klise.
Art Direction by Lindsey Chandler.
08

