Salt Life

01

Blood, Sweat, and Tears
Andrew Bushell has been making sea salt for seven years. For an entrepreneur and small business owner, that’s nothing to scoff at. But it is nothing, he says.
Father Andrew is a Roman Orthodox Catholic monk. He learned to make salt while living, working, and praying at Vatopaidi Monastery on Mount Athos in Greece. “There, we’ve been making salt for 1,651 years.”
“It is the same salt that the Roman Emperors in Constantinople used to eat,” he says. “It was given to them by my brotherhood as part of a tribute every year. It’s a very old thing.”
Father Andrew, who wears rimless glasses and a long salt-and-pepper beard, wasn’t thinking about salt in 2010. That’s when he moved back to Marblehead, Massachusetts, where he grew up, to found a monastery. There was just one problem. He didn’t know how he was going to pay for anything.
The monastic custom, he explained, is to not take any money from the collection plate. A monk is a man with two jobs: to be a cleric and to pay his own way. This is why Dom Pérignon had his Champagne and the Trappists have their jams, jellies, and beer.
One afternoon, soon after returning to the United States, Father Andrew sat on a bench in Fort Sewall park, overlooking the water in Marblehead Harbor. He felt lost. He said prayers. “I was crying—tasting my tears—and I looked at the ocean, and I thought, We could make our salt here.”

He started by borrowing 5-gallon buckets from a nearby Greek restaurant, manually filling them with salt water, and transporting them back to his house in his 27-year-old Volvo station wagon. Today, Father Andrew uses a marine pump to fill a large tank sitting in the back of his dual-rear-wheel pickup truck, which he then empties into two 750-gallon tanks next to his saltern, a small garage-like building where he make his salt. And he doesn’t use just any water. Father Andrew gathers ocean water from anywhere between 14 and 20 different spots off of the rocky coast of Marblehead (all secret), blending them to make each batch of his high-end sea salt. This combination creates the best mineral signature, he says.
To figure out exactly where to get the seawater, Father Andrew tasted water from hundreds of different locations, tracking changes in flavor over time and temperature and season, all of which affect the mineral content of the ocean, he says. If there is a vein of copper or iron somewhere in the seabed floor, that will affect the flavor of the water around it. And that mineral-rich plume of water will move. It will meander. “And that’s part of the trick,” he says. Choosing the best water “requires tasting, trigonometry, and mostly patience.”
Father Andrew’s finished salt is clumpy and moist, with a flavor that changes depending not only on the water collection spot but also on the season. He says that in the summer, when the water is rich with phytoplankton, his salt has a rounder, more complex flavor. In the colder months, it is cleaner. (Father Andrew stops making his salt in the dead of winter due to both the danger of extracting water from freezing, tumultuous seas and the added expense of trying to boil seawater when the outside air temperature drops too low.)
When new customers taste his salt for the first time, Father Andrew says, they are usually “shocked” by the flavor. Shawn Cooney, owner of Corner Stalk Farm, sells hydroponic lettuces at the Boston Public Market, along with one salt: Marblehead Salt Company’s. “We did a taste test of the other salts made in New England, and decided that his stuff was better,” explains Cooney. “It’s that ocean-y flavor profile that it has. The minerality of it. When you put it on something, it just made it taste good. That was the kicker.”
Father Andrew knew something about salt, the only member of the rock family regularly eaten by humans, and an ingredient so common most might assume that it barely needs any consideration. He knew that it could be a dynamic and complex ingredient.
It’s true. And after meeting Father Andrew we—executive editors Molly Birnbaum and Dan Souza—began to dig into the subject, and quickly became obsessed. There are dozens of different types of salt out there. Where do they come from? How are they made? Are they really all that different? When it comes to cooking, does it really matter which salt you use? We set out to demystify this staple ingredient.
02

What Is Salt?
Salt is imperative for far more than cooking. It’s essential for life. For digestion and respiration, for the transportation of oxygen, and nerve transmission. Your heart would not beat without salt. The amniotic fluid that surrounds us as we begin life is salty. Salt has been part of religion and mythology for centuries. It’s associated with fertility. It’s been a driver of commerce and of war. It’s inspired countless engineering feats. Today, millions of tons of salt are used each year in the pharmaceutical industry, in the realms of agriculture and chemistry, in melting ice off of winter roads, and in keeping your pool clean in the summer.
In cooking, salt’s role cannot be overstated. It is used as a flavor enhancer in both sweet and savory dishes. Salt makes sweet foods taste sweeter—this is why some people put salt on their fruit—and bitter foods less bitter. Salt is a tool for manipulating the movement of water in meat and vegetables. As the original preservative, salt makes food safer to eat and store. It keeps dangerous microbes at bay while allowing salt-tolerant bacteria and yeasts to create new flavors and textures in pickled and preserved foods. It’s hard to imagine a meal without salt. And in part, that’s because we don’t have to.
“Salt is so common, so easy to obtain, and so inexpensive that we have forgotten that from the beginning of civilization until about 100 years ago, salt was one of the most sought-after commodities in human history,” writes Mark Kurlansky in his best-selling book Salt: A World History. Technological innovations during the industrial revolution made salt mining more efficient, flooding the market with a supply of cheap salt.
For many years, making salt was a respected profession. “There’s a statue of Roger Conant in Salem,” says Father Andrew. “And his profession was journeyman salter, and he was one of the founders of the city of Salem. It’s important to remember that this was a skill. Because the reality is that today, most Americans have grown up on industrial salt, mined and ground. Salting isn’t seen as a profession . . . it’s a commodity.”

What is salt? The salt that we cook with, or sodium chloride, is a molecule made up of one sodium ion and one chloride ion (though chloride outweighs sodium 1.5 to 1) and known by the chemical formula NaCl. When dissolved in water, sodium chloride separates into positively charged sodium ions and negatively charged chloride ions, particles tiny enough to easily penetrate into meat and plant cells.
In its pure form, all NaCl tastes the same, no matter where it comes from. And salt can come from two sources: seawater and underground deposits formed when ancient oceans dried up. What separates one salt from another in terms of flavor are the amount and variety of minerals (such as magnesium, calcium, and potassium) embedded in the sodium chloride. Sea salt may also contain marine bacteria. Salt’s size and texture, from perfect cubes to pyramid-shaped flakes, are determined by the method of processing.
There are three basic types of culinary salt: table, kosher, and sea salt. Table and kosher salt can be produced by direct mining of salt deposits with heavy machinery, solution mining, where water is piped into salt deposits to dissolve the salt and extract it, or from evaporating seawater. Table salt is crystallized into tiny cubes and often contains additives like potassium iodide and anticaking agents. Kosher salt is coarser and larger than table salt and was originally produced for the purposes of koshering meat under rabbinical supervision. Its irregular texture helps it cling to meat. And sea salt, which comes in a range of colors and textures, is made from seawater. It is often the largest, flakiest salt, containing the largest percentage of minerals and is best used as a finishing salt—sprinkled onto food just before serving to preserve its distinct texture.
03

Sea Salt
When Father Andrew makes sea salt, he uses a traditional process he learned from his Eastern Orthodox brothers on Mount Athos. He won’t divulge the fine details of the process. “Only three members of our brotherhood know the specifics,” Father Andrew explains. But he’s willing to lay out the broad strokes.
First step: select and collect seawater, which he does from up to 20 different locations around Marblehead. This is the base of his salt. “Just like you would have a center chord in a song, like Pachelbel’s Canon in G, for example,” he says. “And then on top of that, we layer the different kinds of minerals, to tease out the main note.” This means adding more seawater, from any number of locations, depending on tide, temperature, and season. He brings it back to his saltern, a small building next to Father Andrew’s house in a quiet residential neighborhood in Marblehead, to blend the water “much like a blended whiskey.” Next, he slowly and carefully reduces the water down. On the holy mountain in Greece, says Father Andrew, they did this in iron pans heated over branches that had fallen off of nearby trees. But in Marblehead, he had to devise a more modern way: enameled cast-iron pots, induction boilers, and rack ovens. It’s a 27-step process, he says, involving measuring, adjusting, and resting. (He claims that periodic resting allows the salt to form its crystals.) The process takes about 2½ days per batch.

On a recent Wednesday morning, we went up to Marblehead to visit Father Andrew, see his production, and taste his sea salt. Father Andrew, dressed in his clerical black wool slacks and sweater, greeted us on his patio with shots of homemade anise-honey liqueur, glasses of water, and jars of his salt. He spooned a small pile of his Athonite Finishing Salt (in reference to Mount Athos) on our palms for us to taste. (Father Andrew also makes 3 flavored salts: fire salt (made with ghost peppers, Thai chilies, and habaneros), garlic salt, and rosemary salt.) Unlike the bone-dry kosher salt one gets in supermarkets, Father Andrew’s sea salt is decidedly moist. And its crystals are surprisingly small for what is meant to be a finishing salt. These qualities can make it challenging to sprinkle his salt evenly onto food. (He recommends only using about half as much of his salt as you would commercial table salt—claiming it has that much flavor.)
And its flavor was intense: The clumpy, bright white crystals dissolve quickly on the tongue, revealing a clean, briny flavor, with no bitter finish. Father Andrew explains that the flavor has a beginning, middle, and end. “And notice how at the end, it goes away. It does what it came to do and then it leaves, which is also very monastic.”

There are many intense-tasting sea salts on the market, and more than one way to make them. At Maldon Salt Company in Essex, England, they simmer concentrated brine until pyramid-shaped crystals form on the surface and sink to the bottom, where they can be raked off and dried in industrial ovens. In Guérande, France, highly prized fleur de sel (flower of salt) forms on the surface of shallow marshes of seawater concentrated through solar evaporation. Once the seawater brine is fully saturated with sodium chloride, all it takes is a gust of wind to evaporate a small amount of additional water at the surface, causing crystals to form, or “bloom.” These delicate crystals are gently raked from the surface before they can settle to the bottom.
And believe it or not, the ocean is not the only place to source seawater for sea salt. In the 1850s, the Appalachian Mountain region of West Virginia was one of the largest sea salt–producing areas of the United States. The source of the seawater was underground: the ancient Iapetus Ocean, a body of water between 400 and 600 million years old, which, through tectonic shifts, became trapped under the mountain range. Nancy Bruns, founder and owner of JQ Dickinson Salt-Works, tapped into her own family’s history in the salt business by once again making sea salt on their property in the Appalachian Mountains in 2013. All Bruns needed was a new well, which she uses to draw the water to the surface of the land.
“Our ancestors used timber and burned coal to make salt on an industrial scale,” says Bruns. “We chose a different path.”
Solar power.
To make sea salt, Bruns lets the water drawn up from the well sit in beds lined with high-density polyethylene, which is stable at high temperatures, in three hoop houses. She lets it evaporate until it’s concentrated down to a 12 percent solution, and then moves it to a nearby greenhouse, where the concentrated brine sits in shallow beds and begins to crystallize. The first crystals Bruns and her crew rake up are the largest.
The finished sea salt is thick and chunky. Like Father Andrew, Bruns recommends that her customers only use half as much as they would a less-artisanal salt. “It has a very bright, bold flavor,” she says. This is due, again, to the trace mineral content. The JQ Dickinson Salt-Works website lists the salt composition as 94.5 percent sodium chloride, 3 percent calcium, 1 percent magnesium, and 1.5 percent trace minerals, including potassium. That might not sound like a lot, but consider that Morton table, kosher, and sea salts all contain less than 1 percent trace minerals (calcium, magnesium, and sulfate, specifically). They are more than 99 percent pure sodium chloride.
But does more minerality mean better salt? Robert Wolke, author of What Einstein Told His Cook: Kitchen Science Explained, writes in his book that “if you evaporate all of the water from a bucket of ocean water (fish previously removed) you will be left with a sticky, gray, bitter-tasting sludge, that is about 78 percent sodium chloride: common salt. Ninety-nine percent of the remaining 22 percent consists of magnesium and calcium compounds, which are mostly responsible for the bitterness.” In fact, sea salt producers often go to great lengths to remove bitter minerals, including crystallizing and separating out bitter calcium compounds from brine, and rinsing finished salt in fresh brine to remove bitter magnesium compounds.
04
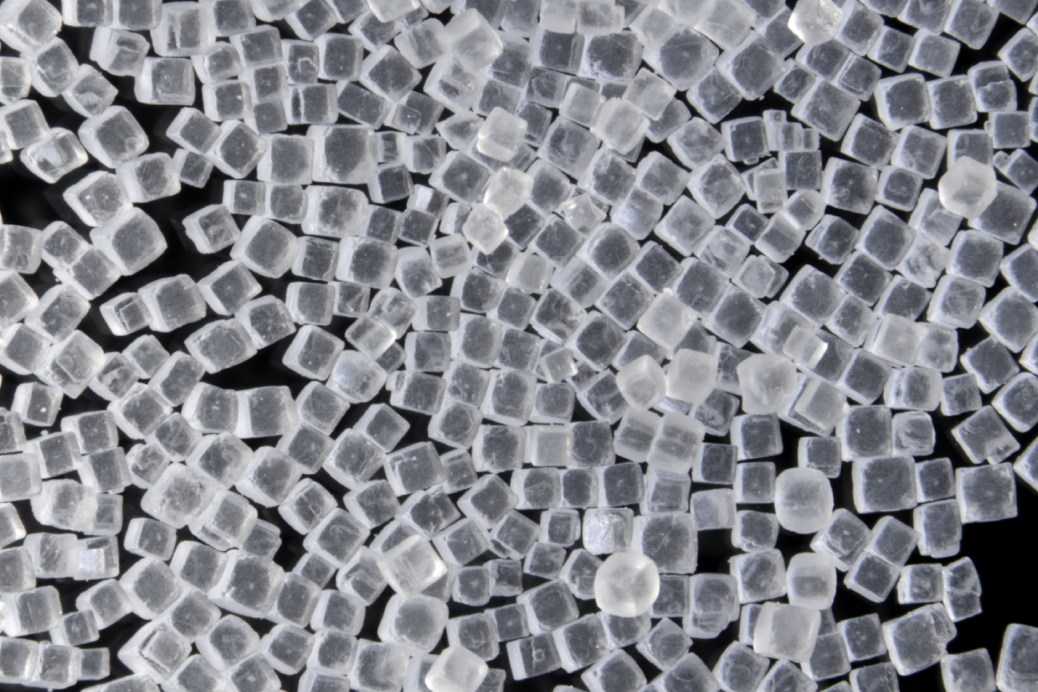
Table and Kosher Salt
Morton Salt has been in the salt business for nearly 170 years. It is best known for its cardboard cylinders of table salt, sporting a drawing of a girl wearing a yellow rain jacket and holding an umbrella. “When it rains it pours,” reads the slogan, a reference to the fact that Morton was the first company to add an anticaking agent to its table salt, making sure it didn’t clump even when it was rainy and humid outside. Clumping can be a big problem for table salt for the same reason it flows so easily from a saltshaker and packs so densely into a teaspoon—it has been crystallized into perfect little cubes. In a humid environment, water molecules in the atmosphere come in contact with salt crystals and stick to the ions on their surfaces. Once wetted, the flat sides of the cubes easily stick together.
According to Harold McGee in his seminal On Food and Cooking, anticaking agents like aluminum and silicon compounds of sodium and calcium, silicon dioxide, and magnesium carbonate “prevent the crystal surfaces from absorbing moisture and sticking to each other.” He adds that “other compounds called humectants may be added to keep these additives from excessive drying and caking.” Flakier salts that can’t nestle together as neatly as table salt’s cubes are less susceptible to clumping.
To make their salt, Morton “starts with drilling wells from several hundred to a thousand feet apart, deep within a salt deposit,” explains company spokesman Denise Lauer. Dry underground salt deposits are found around the world, from Detroit, Michigan, to Africa’s Danikil Desert. “We extract the salt by pumping water into the wells. The salt below is dissolved and you get a brine. We force that brine to the surface through pressure and store it in large tanks.” Morton could, like Father Andrew, boil the brine down in open pots, but that takes a lot of energy, especially for a large industrial operation. Instead, they use a technique called vacuum evaporation. “Then we start to pump it from the tanks into vacuum pans. These are huge closed vessels, ranging from two to three stories high,” says Lauer. The pressure in the vacuum pans is reduced below normal atmospheric pressure so that the boiling point of the brine drops. Steam heats the brine and evaporates the water, leaving salt behind.
And that salt can take different shapes. “From there, we use different forms of processing to get different grain sizes,” says Lauer. To make their bigger, chunkier kosher salt, they run it through a piece of equipment called a flaker. “The salt exits the flaker in a thin sheet of compressed salt, which is then broken up into different varieties of flake salt.” Lauer explains that their table salt isn’t processed through the flaker, but it does go through a screening process to ensure uniform crystal size.

Morton adds anticaking agents to its kosher salt as well, something competitor Diamond Crystal does not do with theirs. According to Diamond Crystal’s website, the company uses “the proprietary Alberger salt process, a method for creating unique-shaped crystals with numerous facets.” In stark comparison to Morton’s goal of creating uniform crystals, the patented Alberger system purposefully forces a wide variety of crystal sizes to form. Fine, clean-cut crystals are first formed by rapidly evaporating brine under violent agitation. These crystals are then transported to a slower evaporating pan where large crystals form at the surface. Eventually the smaller crystals start to adhere to larger ones, producing a mix of shapes and sizes, predominantly hollow pyramidal crystals. This unique crystal structure makes it difficult for the grains to stick together and obviates the need for adding an anticaking agent.
The website description goes on to say, “These facets give your food creations a clean burst of flavor, exceptional adherence, and the distinctive texture recognized by chefs everywhere.” That may sound like flowery marketing-speak, but talk to any chef worth her salt and she’s sure to have an opinion on which brand of kosher salt is best. In The French Laundry Cookbook, famed American chef Thomas Keller writes, “We use a specific brand of kosher salt, Diamond Crystal, because of the size of the grains. We gauge the amount of salt we are using mainly by touch, and we’ve gotten used to the feel of the Diamond Crystal brand.” Keller also admits, “the ability to salt food properly is the single most important skill in cooking.”
So how does one use salt with skill? The first task is picking the right salt for the right application. And that decision comes down almost entirely to the salt’s texture. Kosher salt may be used for a wide range of applications nowadays, but its original use—seasoning meat—is still where it shines brightest. Its flaky, jagged structure makes it easy to grab between fingers and thumb and it sticks to meat with aplomb. (Remember that salt falls evenly from dry fingers but sticks to wet.) Flaky and coarse sea salts also stick nicely to food, making them excellent finishing salts—crystals meant to be sprinkled onto cooked foods to add crunch and pops of salinity. Their high cost makes them prohibitive for seasoning pasta water or making a brine. Table salt, with its fine, tiny crystals, is ill-suited to seasoning by hand, but works well in most savory and sweet dishes where it is measured and then added, as long as there’s enough moisture to dissolve it. (Contrary to common belief, table salt—due to its density—does not dissolve quickly.)
And measuring matters.
Working by mass is easy—a gram of table salt will provide the same salinity as a gram of kosher salt or a gram of sea salt. But each measures differently by volume, due to differences in density and crystal size. Need 5 grams of salt? That’s 1½ teaspoons of Morton Coarse Kosher Salt, 2 teaspoons of Diamond Crystal Kosher Salt, or just 1 teaspoon of table salt. Those are big variations, which is why, if possible, we recommend measuring ingredients by mass.
Salting food early and often during cooking allows it to penetrate into ingredients and season them deeply. How much salt you need to use depends, in part, on how much fat is in the dish, as fat has a dulling effect on taste. In an experiment we performed at America’s Test Kitchen, we found that 80 percent lean ground beef tasted properly seasoned when we added 1 percent salt by weight, while lean turkey breast required just 0.5 percent salt by weight. But the most important consideration when using salt? Taste food often during cooking, and season to taste.
05

How We Perceive Salty
The taste of salt is one of the five basic tastes that humans perceive, along with bitter, sweet, sour, and umami. There’s good reason for each. As we evolved, we developed the ability to perceive these particular tastes because we relied on them to survive: sweet to obtain a high number of calories, bitter to avoid poison, umami for detecting protein, and sour . . . well, sour is a bit trickier, says Gary Beauchamp, emeritus director and president of Monell Chemical Senses Center in Philadelphia, but there must be some reason. The most likely? To detect ripeness in fruit. Our ability to detect the taste of salt, of course, was developed because “sodium is a requirement for life,” says Beauchamp. “Every species will die without enough sodium.”
It is commonly thought that we can perceive these five tastes via individualized taste receptor cells within our taste buds, on our tongue and in our mouth. And that there are taste buds earmarked specifically for bitter, sweet, sour, umami, and salty tastes.
Not quite.
Each individual taste bud in the mouth and on the tongue contains about 100 different cells, which are divided into different groups based on different tastes. “The sweet and bitter cells have specific kinds of receptors on the outside,” says Beauchamp. So when a sweet molecule comes along, it reaches in and fits into a particular part of the cell—like a lock and key. This causes the receptor to change shape, therefore sending a signal to the brain. The message to the brain is: sweet.
And there are many different keys that fit the sweet lock. There are natural sugars like fructose, glucose, and sucrose. There are at least 20 to 30 compounds found in nature, independently evolved in plants, which taste sweet but are not sugars. And of course there are artificial sweeteners.
But for salt, it’s different.
The salt receptors on our taste buds don’t work like a lock and key, explains Beauchamp. Instead there is a hole in each salt taste cell, one that sodium ions can fit in and set off a response.
And this sodium channel is very specific to sodium. “As a consequence of this, there is nothing else that is purely salty, other than sodium chloride,” says Beauchamp. “No one has discovered an aspartame of salt.” Though that doesn’t mean companies don’t try. There are a number of low-salt and no-salt alternatives on the market that substitute potassium chloride for some or all of the sodium chloride. Potassium chloride does have a salt-like taste (how potassium chloride can taste salty is a still a mystery), but it’s also an unpleasant taste. When our colleagues at Cook’s Illustrated ran these alternatives through their rigorous taste-testing process, they found that salt substitutes that replaced no more than one-third of the sodium chloride with potassium chloride were acceptable in many savory and baking applications, while those that contained more than that were bitter and unpleasant.
06

A Healthy Dose
And that, right there, poses a challenge for nutritionists who long for a viable salt substitute. The U.S. Food and Drug Administration recommends consuming no more than 2,300 milligrams of sodium per day (about a teaspoon of table salt). The average American, however, eats around 3,400 milligrams. Where does that sodium come from? According to the USDA’s What We Eat in America report, 77 percent of the sodium consumed by Americans comes from processed and restaurant foods, 6 percent from salt added at the table from the shaker, and just 5 percent from home cooking. The remaining 12 percent is naturally occurring in food.
Not every study on sodium and health finds that too much sodium correlates with cardiac problems, but the big health organizations, like the American Heart Association, are clear in their recommendation: Cut the salt. Research (including Beauchamp’s) has shown that how much we like the taste of salt is influenced, at least in part, by how much we are exposed to it. And therein lies a potential path to a lower-sodium diet. Beauchamp explains that “when people are placed on low-sodium diets, their sensory response to salt shifts downward.” Research demonstrates that subjects switched to a low-sodium diet adjusted to the new level—and found it sufficiently salty—after about three months. The FDA is paying attention to this research. Last year it issued draft guidance with voluntary sodium targets for food manufacturers in the hopes of gradually lowering sodium in the category where Americans consume the most. Beauchamp thinks it could work. “The entire population could be shifted downward,” he says. “No one would be happy. But we could move down to a healthy level.”
If salt’s only role were to make food taste seasoned, then shifting to a low-salt diet (and waiting months for food to taste good again), or putting up with the unpleasant taste of salt alternatives, might be a way forward. But salt does so much more than season.
For one, salt—or the sodium ion itself—is a great inhibitor of the taste of bitter. “One reason sodium is so ubiquitous [is because] it’s added to get rid of unpleasant bitterness of vegetables and other [processed food] things,” says Beauchamp. “It’s cheap and effective.”
In traditionally fermented pickles, sauerkraut, and kimchi, salty brine creates a selective environment where salt-tolerant lactic acid bacteria (LAB) gain a competitive advantage over salt-intolerant microbes that can cause spoilage. LAB produce lactic acid that lowers the pH of the pickle and extends its shelf life.
Salt strengthens gluten and improves texture in bread dough. It tenderizes scrambled eggs and keeps brined chicken breasts juicy, by altering their protein structure so it can’t squeeze together as tightly when cooked. lt suppresses the bitterness in dark, leafy greens (something we should all eat more of). Adding salt to food can actually make it more aromatic by creating a less hospitable place for aroma molecules to reside and thereby increasing their volatility.
And salt plays a key role in creating some of our favorite foods, from cured egg yolks, gravlax, and anchovies to soy sauce, cheese, sausage, and aged hams.
07

Covenant of Salt
After spending the morning with Father Andrew and his salt enterprise in Marblehead, we ducked into the Barnacle restaurant to grab lunch. We settled into stools at a counter at the back window, where we could look out over overcast Marblehead Harbor. Throughout our day together Father Andrew had effortlessly shifted between the topics of salt and theology, often offering analogies between the two. After we ordered our clam chowder and fish and chips, he offered his most direct comparison.
“The role of salt in food should be like the role of the clergy for the people. It should be bright. It should bring out the best. And it should leave no trace.”
He then went on to discuss his forthcoming trip back to Mount Athos for the prayer services and activities around the week of Easter, Holy Week. When he described the services planned for Saturday, the day before exultant Easter Sunday, his eyes welled with tears.
Our food arrived and Father Andrew started to reach for the saltshaker to dust his fries. “Agh,” he said with a sigh, “I should have brought my salt.”
08

Back to the Test Kitchen
[Ed note: After executive editors Molly Birnbaum and Dan Souza reported and wrote their salt story, Dan, associate editor Tim Chin, and test cook Sasha Marx began developing salt-focused recipes. The following is from Dan’s perspective.]
When I visited him in Marblehead, Father Andrew offered to show me two of the dozens of locations from which he collects seawater, on the condition I wouldn’t reveal them in the story. I, of course, agreed. After we left Father Andrew’s house and before heading back to Boston, we stopped by one of the spots and I filled five 18-quart containers with fresh, clear Marblehead seawater. (I, regrettably, didn’t have any boots with me so I was forced to go in barefoot, a reluctant mid-March polar bear wade of sorts.) After diving into this salt story I was anxious to try my hand at making sea salt at home. Sure, I wouldn’t have Father Andrew’s blended water or his years of firsthand experience (or centuries of institutional knowledge) to draw on, but I do have access to enameled Dutch ovens. And science.
During my research I learned that the rate of water evaporation has an impact on the size of crystals that form when making salt. Generally speaking, slower evaporation leads to larger crystal structure. It made sense to me. When I freeze ice cream base quickly in a high-end ice cream machine, I get small, undetectable ice crystals. In a slower home ice cream machine, the crystals, which form more slowly, are coarser.

After filtering the Marblehead water through a coffee filter (to remove silt and a few stray pieces of seaweed) I tested this theory. I added a gallon of seawater to each of three enameled Dutch ovens (boiling salt water for an extended period of time is highly corrosive, so it’s important to use an enameled pot) and reduced them down at different rates. For one pot, I basically boiled the water dry. For the second, I boiled the water down to 2 cups (the point at which I saw some tiny crystals form at the surface and a little sediment appear at the bottom), then transferred the pot to a 400-degree oven to finish the evaporation at a more controlled rate. And for the third pot, I again boiled it down to 2 cups and then transferred the pot to a 300-degree oven for an even slower finish. In all of the batches I got a mix of crystal shapes, but the slowest method produced the largest crystals. I even got a few of the pyramid-shaped flakes that Maldon sea salt made famous.
Texture? Check.
Flavor? Bitter.
As Harold McGee writes in On Food and Cooking, the bitter components of sea salt are usually the chloride and sulfate salts of magnesium and calcium. He notes that the salt industry deals with each of these components a bit differently. These calcium salts are less soluble in water than sodium chloride, so they will precipitate (come out of solution and sink to the bottom of the pot) before sodium chloride does. They can then be raked away and discarded before sodium chloride crystals form. I noticed that when my brine had reduced to about 2 cups and just before it started to form sodium chloride crystals, a layer of fine silt formed on the bottom of the pot. After carefully pouring off the top brine I tasted the sandy residue. It didn’t dissolve on my tongue the way sodium chloride does (indicating that it was less soluble than sodium chloride), and it tasted very bitter. I had a hunch it was calcium salts so I ditched the sludge and kept cooking the remaining brine down until it fully crystallized. This batch of salt was less bitter than my previous attempt, but some bitterness still lingered.
To get rid of magnesium salts—the other bitter culprit—as well as other surface impurities, salt producers will often wash the salt with a fully saturated solution of pure salt. The solution is full of sodium chloride, so it won’t dissolve any more of that, but it will gladly dissolve the magnesium you’re trying to get rid of. So I tried that. If the thought of washing salt (and having it not just dissolve) sounds weird to you, you’re not alone. I trusted the science, but I still expected my precious homemade salt to disintegrate when I poured fresh brine on it. But, amazingly, that didn’t happen. After rinsing my salt in brine I’d made with Diamond Crystal kosher salt (to avoid adding iodine and anticaking agents found in table salt), I fully dried it out in the oven. I was left with a really nice sea salt. It had a light, crunchy texture and clean, round salinity. It’s great sprinkled on grilled steak, fish crudo, or nice cultured butter. It would even make a special handmade gift. I’m excited to try my recipe for Homemade Sea Salt with water from other ocean sources and compare their flavors.
While I was (literally) up to my knees in seawater and sea salt, associate editor Tim Chin and test cook Sasha Marx dove into salt-focused recipes of their own.
Tim’s Crispy Pan-Seared Fish with Piquillo Pepper Sauce highlights the ability of salt to impact the movement of water. The fish is quick-cured for just 45 minutes with a dusting of salt and sugar. As the salt dissolves in the moisture on the surface of the fish, it creates a high concentration of sodium and chloride ions. Moisture in the fish is then driven by osmotic pressure to exit the flesh. At the same time, the salt and sugar diffuse into the fish to provide seasoning. In true curing, which uses far more salt and can take days, the goal is to remove enough moisture to hinder the growth of harmful microbes. Here, we remove just enough moisture to slightly firm the fish. To cap it all off, Tim developed a low and slow searing technique that delivers ultracrispy skin and moist, tender flesh.
Anyone who’s soaked chicken breasts in a salt solution for an hour before cooking them knows the transformative power of brining. Lean cuts of poultry and meat cook up remarkably juicy and well seasoned. In his recipes, Sasha shows that a brine can be used to success on vegetables as well. We love grilling whole vegetables like carrots, asparagus, and zucchini, but getting even, deep seasoning is a challenge. Kosher salt easily adheres to raw meat, but merely bounces off of raw vegetables without a thorough coating of oil first. And even if it does stick, the seasoning impact is purely superficial. By soaking young carrots and thick asparagus spears in an 8 percent salt solution (similar to the brine strength we often use for meat and poultry) for just 45 minutes—the time it takes to prep and preheat the grill—we get deep salt penetration and incredibly flavorful grilled vegetables. Check out Sasha’s Brined Grilled Carrots with Cilantro-Yogurt Sauce and Brined Grilled Asparagus with Preserved Lemon Aïoli. Don’t have preserved lemons on hand, nor the month required to traditionally ferment them? Try his recipe for Quick Preserved Lemons, which uses a hot salt-sugar brine and is ready in less than 24 hours.

Sasha’s final recipe is a simple and flexible technique for rescuing unused fresh herbs from certain death in the crisper drawer. Sasha rubs together chopped fresh herbs—everything from dill and basil to chives and makrut (kaffir) lime leaves—with flake or coarse sea salt and lets it sit at room temperature for a couple of days. During that time, salt draws moisture from the herbs, dehydrating them with no heat. The result? Fresh Herb Finishing Salts that actually taste like fresh herbs and that can be stored for months, ready to top meats, fish, vegetables, or even the rim of your cocktail glass.
Field photography by Kevin White.
Test kitchen and styled food photography by Steve Klise.
Art direction by Lindsey Chandler.

